Expert Guide to Medical Device Software Development in 2025
Updated 13 Mar 2025
20 Min
625 Views
In 2020, we started a partnership with a US manufacturer of medical IoT hardware, particularly ECG heart monitoring devices and pulse oximeters. The organization approached us as an experienced healthcare software vendor to help them with medical device software development to expand their range of digital services and provide a platform integrated with the company's pulse oximeters and IoT devices. We accepted the client's request and successfully launched the product.
We, at Cleveroad, know all the nuances of medical device software. Therefore, in this article, we will tell you how to develop software as a medical device, what types and complexities such solutions have, and disclose other critical aspects you should know about.
What Is Medical Device Software Development?
Medical device software development focuses on designing applications that work within or alongside medical devices to support diagnostics, monitoring, and treatment. These solutions range from embedded software in medical equipment to real-time monitoring tools and AI-driven diagnostic systems. Given their critical role in patient care, such software must adhere to strict regulatory standards to guarantee accuracy, reliability, and safety.
By leveraging advanced technologies, medical device software enhances device performance, optimizes data processing, and supports clinical decision-making — ultimately leading to better patient outcomes. Regardless of the physical device for which the software is created, it performs medical functions such as diagnosis, treatment, monitoring, or recommendations for medical use.
Medical device system development is in demand in modern medicine due to growing usage of wearable devices, smart devices, and IoT-enabled systems. The work mechanism of such software may vary depending on the type of equipment and its specific functions. However, it has a few standard steps that are the same for most systems. Let's examine how medical device software typically works:
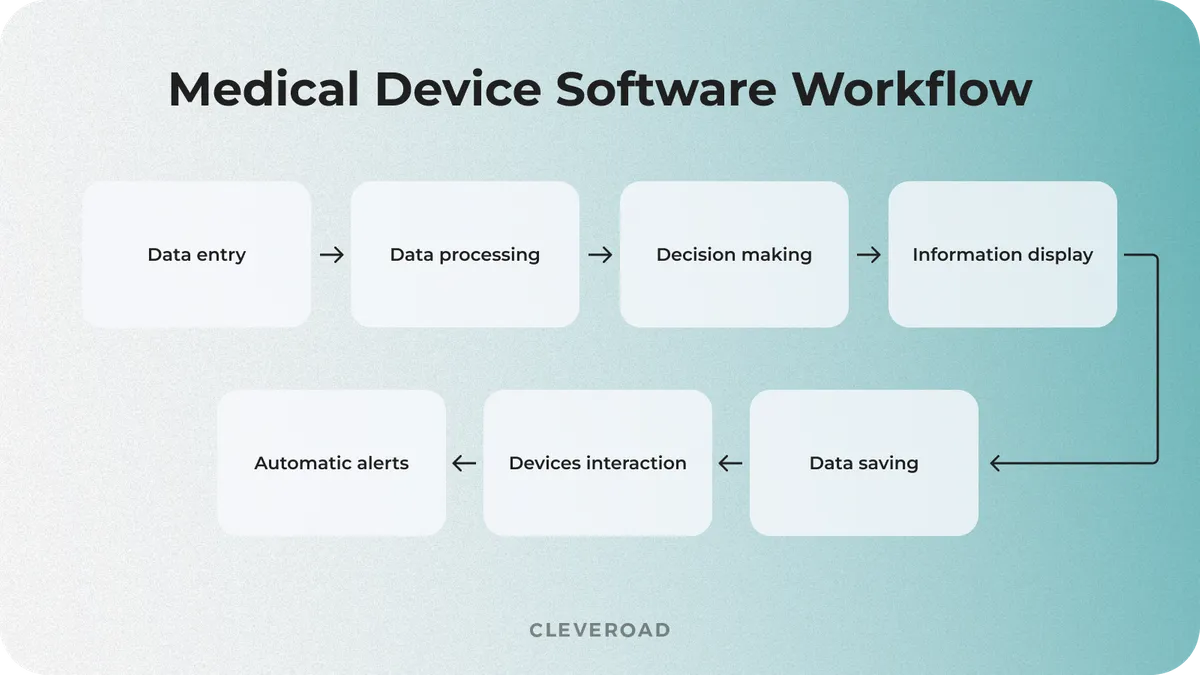
How medical device software works
Types of medical device software
Medical device software can be divided into three main types that vary based on technology, function, and regulatory framework:
- Embedded Medical Software
- Software as a Medical Device (SaMD)
- Medical Device Data Systems (MDDS)
Embedded Medical Software generally runs on hardware medical devices and is embedded with the hardware. It guarantees real-time safety so that devices such as pacemakers, infusion pumps, and MRI machines can do their work autonomously without depending on outside systems. However, such software needs to meet the strict safety and performance standards required of medical devices, given manufacturers’ responsibilities to minimize patient risk.
Software as a Medical Device (SaMD) is a type of software that itself falls under the category of a medical device and does not require dedicated hardware, i.e., it runs on general-purpose hardware. This covers AI-powered diagnostic tools, mobile health apps, cloud-based analytics, and diagnostics for critical care. SaMD faces the same regulatory scrutiny as that applied to physical medical devices.
A software developer working on SaMD for the medical device software development needs to think about and ensure compliance with global regulatory frameworks such as the FDA, MDR, or ISO 13485.
Medical Device Data Systems (MDDS) are software solutions that collect, transmit, or store medical data without processing or transforming it for clinical decision-making. These systems connect embedded devices to and within healthcare IT ecosystems, creating a seamless data flow between medical equipment and electronic health records (EHRs). Regular software maintenance is needed to ensure data integrity, cybersecurity, and compliance with changing regulations.
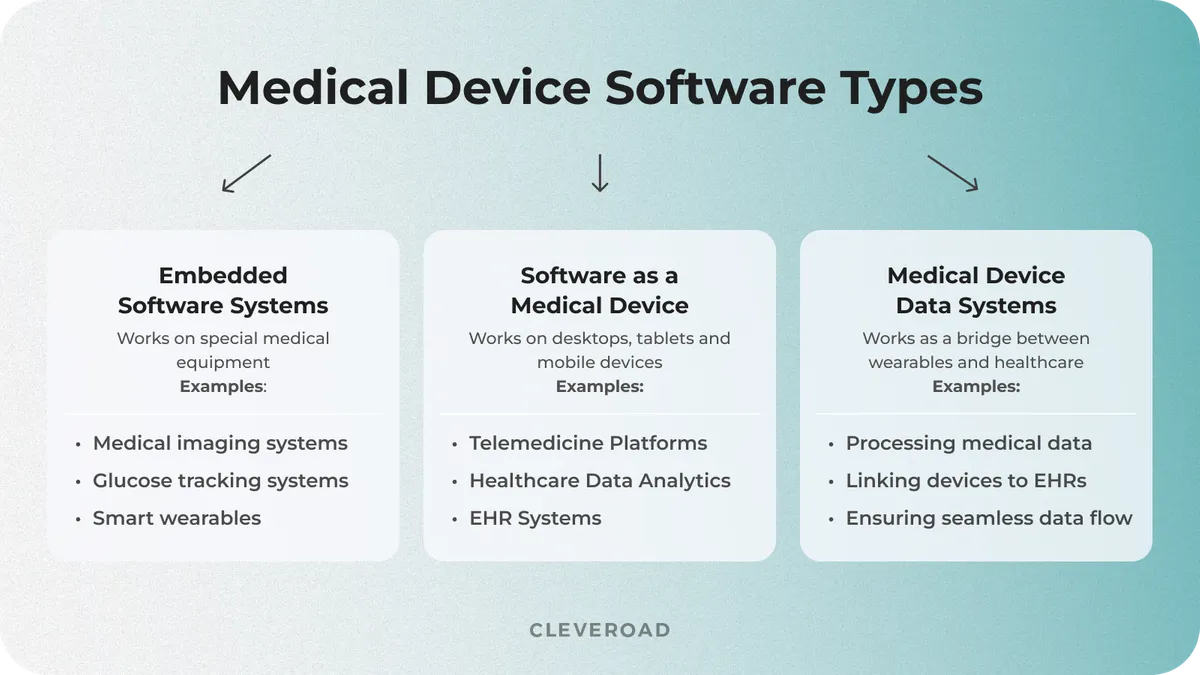
Medical device software types
Benefits of Medical Device App Development
If you are a startup or a small or medium business looking for innovative solutions, custom medical device app development can be highly beneficial. Below are some significant advantages of using software in the medical device industry.
Increased efficiency and accuracy
Solutions for health equipment transform healthcare by automating complex tasks, minimizing human error, and enhancing the accuracy of diagnostics and treatments. AI-driven imaging tools, for instance, can identify anomalies in scans with remarkable precision, while real-time monitoring systems instantly alert doctors to critical shifts in a patient’s vital signs.
Well-designed software enables healthcare professionals to seamlessly access and interpret real-time data, leading to faster, more informed decisions. Additionally, automating data processing reduces administrative burdens, allowing medical teams to dedicate more time to patient care.
Quicker software safety and compliance
Regulatory compliance plays a vital role in medical device software engineering, directly influencing patient safety and data security. A well-structured engineering approach ensures alignment with global industry standards, minimizing vulnerabilities and enhancing software reliability.
Ensuring software safety means that applications function dependably, preventing harm or system failures and mitigating risks for both healthcare providers and patients. Skilled software engineers integrate robust security measures and compliance strategies tailored to industry regulations. By adopting a focused and methodical approach to design and development, your organization can produce high-quality software while efficiently managing regulatory challenges.
Seamless integration with clinic’s tech ecosystem
Medical device software development supports seamless integration among medical devices and health IT systems, like EHRs, lab systems, and other clinical platforms, allowing for fast and efficient access to critical patient information, like ECG results. Properly integrated software for medical device makers supports interoperability, allowing devices to communicate with each other and with health infrastructures with ease.
A medical device is defined not only by the equipment but also by the way it is incorporated in digital health environments, enabling diagnostic, therapeutic, and monitoring functions. With the use of a properly designed medical device software, your hospital or clinic can maximize data exchange, enable real-time decision support, and improve patient outcomes. With your business investing in a robust software solution for health gadgets, it becomes more competitive in terms of accessibility, interoperability, and connectivity in the healthcare setting.
Why will medical device integration be beneficial for your facility? Read our guide and discover!
Scalability and long-term investment
A robust medical device software product can evolve alongside your medical facility's needs and healthcare industry development. Scalable solutions are essential for medical startups and SMBs, as they enable growth without requiring costly system overhauls.
A well-designed medical device software solution ensures scalability and architectural flexibility, allowing you to integrate smoothly and support increasing volumes of information as your company grows. With no sacrifices in performance or security, you can expand functionalities as you go through the medical device software development process. High-quality medical software can be a valuable investment that drives success in an ever-changing healthcare environment.
What You Should Know Before Medical Device Software Development
Before starting medical device software development, there are several essential aspects you should take care of.
Data security and privacy
Medical devices often collect and process sensitive medical data such as patient histories, test results, personal data, etc. A security breach of this data can lead to confidential information leakage, patient identification, or even the possibility of unauthorized access to the device.
To ensure robust healthcare data security, your software as a medical device developer should use powerful data encryption certificates that equip the software with strong authentication (e.g., passwords, biometrics) and access control (differentiation of rights). Moreover, you should consider the possibility of training your staff through educational materials, such as tutorials, webinars, user guides, etc. and initiate regular security audits.
We use a variety of approaches to ensure the protection of Personal Health Information (PHI), including access control, automatic logouts, activity tracking, secure data storage, industry-standard data encryption and secure data transmission under HL7, HTTPS, and WebRTC.
Regulatory compliance in your region
Medical devices are subject to strict regulations that vary in various regions. For example, they include:

Regulatory compliance for your region
Certification and regulatory challenges in medical device software development requires a complex approach to software development life cycle, risk management and software verification and validation activities. To overcome this challenge, contact a software provider who specializes in creating regulatory-compliant medical software. Your IT partner will research your region's medical regulations and standards and consider them during software development for medical devices. Noncompliance with these regulations when developing medical device software can result in denial of certification, fines, and legal problems.
Our team has hands-on experience in developing legislation-based software development, ensuring compliance with various healthcare regulations, such as HIPAA, PIPEDA, GDPR, FDA, FHIR, DICOM, etc.
Integration with Quality Management System
Some healthcare regulations (FDA, MDR, CMDR, TGA, etc.) require building of medical device software under a certified Quality Management System (QMS). Such a system is also critical for liability considerations. If there is an incident involving a medical device, having a certified QMS will indicate that you have taken all necessary measures to ensure the quality and safety of the medical device.
Furthermore, if you plan to provide a SaaS-based medical device solution, QMS is required for market acceptance. Without confirmation that the medical device has passed QMS validation, selling your solution to healthcare providers will be difficult or impossible.
By the way, we, at Cleveroad, had experience developing a Quality Management System for our US-based client (particularly, medical device consulting firm).The development team created a B2B SaaS solution that automates core processes and document flow needed for medical device’s production FDA and ISO certification. QMS meets all US regulations and the FDA 21 CFR 820, 21 CFR 11, ISO 13485:2016, and MDSAP requirements for medical devices and their production.
Here's an opinion of our client about their successful cooperation experience with Cleverod on Quality Management System engineering:
Breanne Butler, Project manager at Prime Path Medtech™ leaves feedback about cooperation with Cleveroad
Interoperability with medical systems
Medical devices and systems may exchange information using different communication protocols and data formats (e.g., HL7, DICOM, MDC). Additionally, devices might communicate via Bluetooth, Wi-Fi, or proprietary protocols, each tailored to specific requirements. Incompatibility can create challenges when integrating medical device software with other systems, especially if they were developed by different vendors or on various technology platforms. Interactions between devices and systems require compliance with specific standards to ensure data is exchanged correctly and without errors.
To address this issue, your software provider will begin the medical device software development process by conducting interoperability tests between your medical software and other systems. In case of inconsistencies, they can help you with integrating HL7, DICOM, or IHE - standards that ensure interoperability and allow for efficient data exchange.
Technical complexity
Medical device system development is technically complex due to the unique requirements and features accompanying medical applications. Medical devices and systems require processing large amounts of data, implementing complex algorithms, and ensuring high performance and reliability. In addition, such devices often operate in critical medical scenarios where errors and failures can severely affect patients' health.
The key to successfully overcoming this and other complexities is to work with an experienced software provider experienced in medical device software development. A reliable IT partner can help you through the research and planning phase, identify requirements, and build medical device software that meets all industry regulations.
Discover how to solve your tech challenges during medical device solution creation through our healthcare software development services
How to Develop Software as a Medical Device
Medical device software development is a complicated process that requires a deep understanding of the peculiarities of such solutions. Let's consider the main steps you should pass to successfully deliver software as a medical device and integrate it into your business processes.
Step 1. Understand regulatory requirements
Medical device system development requires a deep understanding of the regulatory requirements. So, studying this issue before creating such a solution is crucial, whether it is hospital or pharmacy management system design, etc. The main regulations governing medical device software are the FDA for the US and the EMA for the EU. Let's explore their requirements:
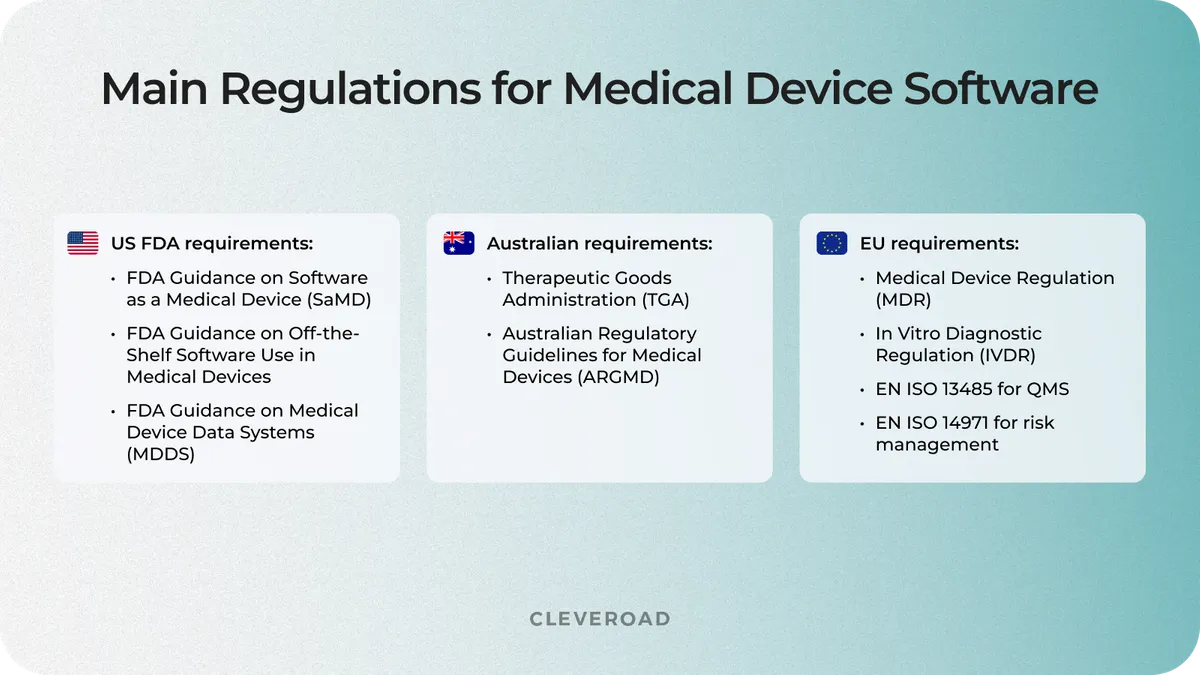
Main regulations for medical device software
Compliance with these regulations is mandatory for medical device software authorization. Therefore, your IT vendor should carefully review and follow these regulations throughout the product development and certification.
Step 2. Do market research and clarify user requirements
Market and user requirements research is another significant step in medical device software and app development. Start with analyzing existing products to determine their advantages and disadvantages. You can also study trends and innovations in the medical field.
Moreover, understanding the needs of the target users is also essential. You can talk to your employees to understand their pains and needs that could be addressed by such type of software.
Creating software usage scenarios also helps better understand the application context and user needs. Your software provider can help you with market research, the business environment and processes analysis. Then, your vendor will form product requirements to address these needs and determine which points are critical for successful medical device app development.
Step 3. Find an experienced IT vendor
Finding an experienced tech vendor is critical for successful medical device software development. To choose the best IT partner, you should conduct market research and determine your selection criteria, then review the portfolios and references of potential vendors. Make sure they have experience in the medical industry, meet healthcare industry standards and regulations, and have certifications (e.g. AWS Security Certificate, A-CSPO certificate, etc.) to prove their expertise. Also, discuss their approaches to development and data safety and negotiate a contract with clearly defined terms and conditions. Choosing an experienced vendor will ensure a successful and secure medical device app implementation.
Here is our client's opinion left on Clutch on the successful cooperation with Cleveroad:


Step 4. Go through the planning stage with your vendor
The planning stage is a key for successful software development for medical devices. During this phase, your software provider conducts a detailed analysis of all medical software details, including user needs, functional and non-functional requirements, 3rd-party integrations, and implementation plan. Developers also consider system architecture to ensure effective integration with other medical systems and high performance and reliability. One example of a suitable architecture for such solutions is the Microservices Architecture, which involves breaking down the application into smaller, independent services that can be developed, deployed, and scaled individually.
Also, at this stage the Solution Architect defines crucial security requirements to ensure solution and medical data safety. The specialist analyzes and implements all domain regulations requirements depending on your region and takes into account security measures such as data encryption, user authentication, audit trails, and secure communication protocols.
Design is another essential part of the planning stage. The team of UI/UX designers works on developing the user interface, considering usability and convenient navigation for the medical staff. Passing the planning phase allows the team to determine the medical device software's engineering timeline, project’s estimate , and the most important tech details, resulting in faster and more efficient medical solution development.
Step 5. Development and integration
Medical device software development involves building core modules and features during two-week sprints. Developers consider regulatory requirements, ensure data security and confidentiality, and focus on flawless medical device integration with EHR and other necessary healthcare software. Depending on your needs, your software provider can offer you two main approaches to medical device software development:
If you want to build SaaS-based medical device software, it’s better to start with the Minimum Viable Product (MVP) version. MVP development implies creating the minimum required functionality of the system before it can be released. This approach lets you quickly introduce medical device software to potential users and gather feedback for improvements and updates. Later, you can ask your vendor to gradually extend the product's functionality based on user feedback and new requirements.
In case you need to integrate medical device software into your healthcare systems, the team will develop a Proof of Concept (POC). This approach allows testing the feasibility of a successful solution’s integration to ensure that medical device software functions properly and is compatible with your business environment. In this case, developers build a limited prototype to demonstrate basic functionality and interoperability with one of your systems.
Here are the examples of systems your medical device software can be integrated with:
- Remote Patient Monitoring (RPM)
- Electronic Health Records (EHR)
- Hospital Information Systems (HIS)
- Pharmacy Management Systems
- Clinic Management Systems, etc.
The tech stack for medical device system development can include the combination of several technologies (based on our experience):

Medical device app tech stack
Step 6. Implementation and monitoring
Once medical device application development and testing are complete, your software vendor will prepare the product for release, including creating a user guide, assisting with staff training, and providing you with all documentation. Your IT partner will also help implement or integrate the medical device software.
Once the system runs, you should continuously monitor its performance. Monitoring allows you to identify possible problems and deficiencies that need to be corrected. It is equally essential to keep the medical device system compliant with industry regulatory requirements and perform mandatory updates to ensure safety and security.
Step 7. Support and improvements
The software provider offers reliable technical support to medical device app users. The team of specialists promptly responds to requests and helps solve technical issues, minimizing possible downtime. In addition, the provider regularly releases updates and fixes that eliminate bugs, improve functionality, and ensure product security.
Your software provider will also help you with updates and improvements based on user feedback. This process keeps your medical software up-to-date, ensuring that it is reliable and meets the high standards of the medical industry.
How to Find the Right Software Development Partner for Healthcare Development?
Selecting a reliable software provider is a key factor influencing the success of medical device software development. Let's look at the main points you should consider when looking for an IT partner to make the best choice.
1. Experience and expertise. Ensure that the company has a background in medical software development and relevant technical skills. The IT partner should understand the requirements of regulatory organizations such as the FDA and EMA, and follow medical standards and regulations, such as HIPAA, GDPR, PIPEDA, etc.
2. Portfolio and references. Examine the software development partner portfolio and reviews from their clients. It will help you assess the quality and success of past healthcare projects and determine if their experience matches your requirements. To find real reviews, you can use platforms like Clutch.
3. Security and privacy. Ensure the software provider you choose follows security and privacy regulations for patient data and medical information. Also, check whether the selected company signs a Non-Disclosure Agreement (NDA).
4. Development methodology. Familiarize yourself with the methodology used by selected software providers. Agile methodology will be the best option for medical devices software development, as it provides higher flexibility and better quality. We adhere to Agile and Scrum methods, promoting frequent communication, rapid changes adoption, and high customer engagement.
5. Collaboration options available. Consider if the software vendor can offer you the cooperation model you need to ensure the company can provide the required employees. Cleveroad offers flexible cooperation models: time & material, dedicated team, fixed workscope and team augmentation.
Finding qualified software as a medical device developer in your region may be challenging, so it’s good to consider outsourcing. By outsourcing software development for medical devices, you can make up for the lack of experienced professionals, as you will have access to talented experts worldwide.
What's more, you can reduce costs by choosing the cooperation model suitable for your needs:
Outstaffing
This collaboration model allows you to hire several specialists from the team of an outsourced software provider to complement your in-house team. For example, if you need 1 developer, 1 QA engineer, and 1 designer for your medical device software development, you can ask the vendor to provide you with these employees. This way, you can get the missing healthcare solution creation expertise quickly, optimizing the cost for hiring and maintaining in-house staff. If you choose this model, you are responsible for the project and development control.
Cleveroad also provides qualitative IT staff augmentation services. Our team of highly skilled web and mobile specialists is ready to support your medical device app development initiatives. Beyond development, you can also collaborate with seasoned CTOs, Business Analysts, and Solution Architects to lead your digital transformation and drive your healthcare product’s success with strategic expertise.
Dedicated development team
This cooperation model allows you to hire an entire development team to work together from the planning phase to the release. With dedicated development team services, you can outsource your medical device solution development to experts with minimal involvement in the development process. If you choose this model, the provider is responsible for the delivery project and product quality. You monitor the quality and project deadlines. Moreover, choosing this model allows you to significantly reduce costs during medical device app creation, as you do not need to spend money on administrative needs.
Cleveroad renders dedicated development team services customized to fit your business needs during medical device app development, ensuring you work with specialists who have the right industry expertise. Our teams integrate smoothly into your workflows, maximizing efficiency and delivering scalable, high-performing solutions.
Cleveroad Experience in Developing Medical Device Systems
Our team has 13+ years of experience creating healthcare and telemedicine solutions. Now, we would like to present to you some examples of medical device solutions that we have built for our clients:
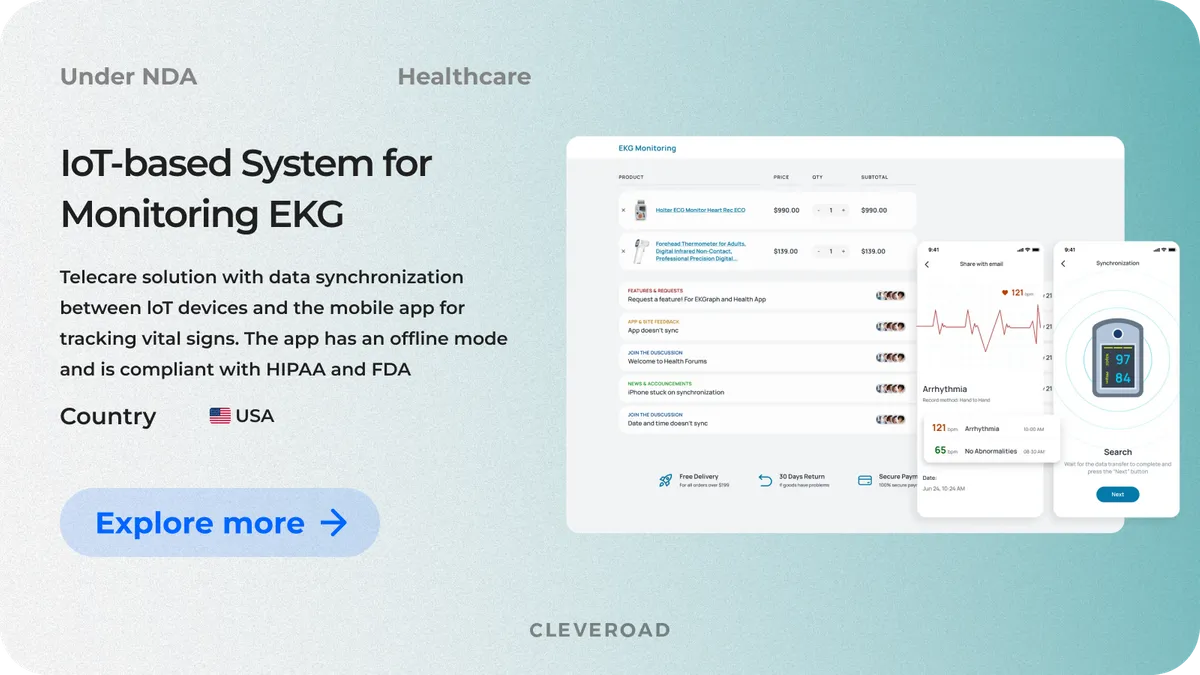
IoT-based System for Monitoring EKG and Blood Oxygen Level
Our client is a US-based manufacturer that provides medical practitioners with IoT devices, namely ECG heart monitors and pulse oximeters. The company approached us to develop an IoT system for tracking ECG and checking oxygen concentration in users’ blood from mobile devices in real-time.
To address the client's business needs, we:
- Built a mobile telecare app to track heart rate and blood oxygen levels from a user's smartphone
- Ensured stable connection between the application and the client's IoT devices via Bluetooth
- Ensured that the software meets the requirements of the 510(k) FDA Medical Device Registration and is HIPAA compliant to allow the app to store and process Personal Health Information (PHI) data
The solution consists of iOS and Android mobile apps connected to IoT devices via Bluetooth. The system can interact with one of the client's devices (heart rate monitor or EKG monitor) or both simultaneously. Among the key functions of the solution are data synchronization between IoT devices and the mobile applications, personal Health Information from the ECG monitor, the history of all measurements, personal Health Information on oxygen saturation, direct and secure report sending.
As a result, our team seamlessly integrated the client’s IoT devices (such as EKG monitors and pulse oximeters) with mobile apps, delivering a smooth, real-time health tracking experience for users. The solution fully complies with FDA 510(k) and HIPAA regulations, enabling the client to legally offer "home EKG" services in the U.S. and expand into the B2B healthcare sector. Thanks to our tailored approach and quality management, our solution is both reliable and user-friendly: 95% of users' reviews have rated it with "4-5 stars". The app also gets positive feedback from physicians.
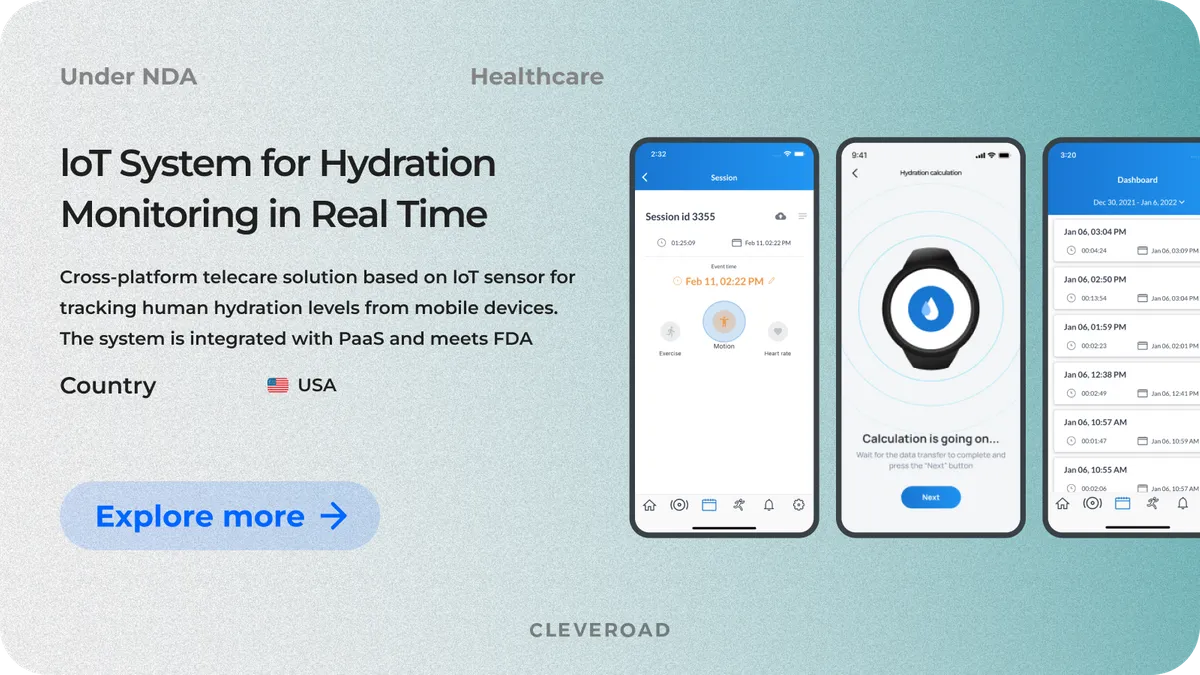
IoT-Based System of Human Hydration Monitoring
Another of our medical projects was executed for a US-based company that provides a solution for tracking human hydration levels. The company approached us for an IoT-based system for hydration monitoring in real time. We helped our client to:
- Create a medical device software and integrated it with the existing hardware IoT product
- Build system architecture complying with 510(k) FDA Medical Device Registration regulation
- Ensure the capability for integration with customer's PaaS for synchronizing and storing data
Key modules of the solution include data synchronization, hydration level measuring via the sensor, and hydration calculation. We continue our partnership with the client to provide ongoing support, upgrades, and maintenance of the developed solution.
As an outcome, after this medical device software development process, the client now has a fully integrated, FDA-compliant IoT-based hydration monitoring system that has doubled its user base while ensuring seamless data synchronization. The solution also paved the way for expanding partnership programs and reinforcing the company’s market presence.
In Conclusion
If you need a professional IT partner for medical device software development, the Cleveroad team is ready to assist you. We have helped healthcare organizations create turnkey products and simplify digital transformation for over 13 years. Our expertise includes the development of EHR, EMR/EPR, patient portals, Hospital Management Software (HMS), telemedicine systems, and other medical software solutions.
By choosing Cleveroad, you will get the following benefits:
- Practical experience in healthcare software development, including EHR/EMR systems, Remote Patient Monitoring solutions, Quality Management Systems, telecare solutions, etc.
- Cooperation with an ISO-certified healthcare software development vendor while implementing ISO 27001 security handling, as well as ISO 9001 quality management standards
- Experience in creating healthcare and telehealth software compliant with industry security and regulatory standards, such as HIPAA, GDPR, FDA, PIPEDA, EMA, etc.
- Expertise in integrating medical software with 3rd-party tools, such as Kareo, Intermedica, Twilio, Vonage, MedlinePlus, etc.
- On-demand services: full-cycle development, from-scratch engineering, IT consulting, legacy software modernization, managed IT services, etc.
- Free Solution Design Workshop to consult our Healthcare subject matter experts as to the development and implementation of medical device software
- Signing a Non-Disclosure Agreement (NDA) per your request to protect intellectual property and maintain confidentiality
- Flexible models for seamless cooperation: Team Augmentation, Dedicated Development Team, Project-Based model
Cooperate with Cleveroad to turn your vision for medical device software development into reality. Together, we’ll create innovative, secure, and fully compliant healthcare solutions. Contact us today, and let’s discuss how our expertise can drive your project’s success!
Order medical device software today
We will provide you with custom medical device software development services for better patient care – contact us now
Medical device software development focuses on designing and maintaining software for medical devices that support diagnosis, treatment, and patient monitoring. As outlined in the definition of a medical device, such software must adhere to strict regulatory standards to guarantee safety and effectiveness. To facilitate compliance, organizations like the International Medical Device Regulators Forum offer global guidelines that help streamline medical device development and regulatory approval.
SaMD (Software as a Medical Device) refers to software designed for medical purposes without being embedded in a physical device. A medical device software development company plays a critical role in ensuring regulatory compliance and implementing a robust software risk management process to maintain safety and effectiveness.
Examples of SaMD include AI-driven diagnostic tools, mobile ECG monitoring apps, and cloud-based imaging analysis, all supporting a wide range of medical applications. As software development in the medical field continues to advance, SaMD is becoming a key driver of innovation in healthcare.
Developing software in medical applications starts with defining its purpose—whether it’s software that helps with diagnostics, patient management, or telemedicine. Ensuring smooth integration of software with existing systems is essential, especially when it’s part of a hardware medical device.
Selecting the right medical device software depends on the types of software for medical use, including EHR systems, telehealth platforms, and remote patient monitoring solutions. Regulatory compliance must also be a priority to ensure safety and effectiveness.
One of the key benefits of SaMD development is its ability to build software for medical applications that enhance diagnostics, monitoring, and treatment. Unlike traditional devices, computer software in SaMD allows for real-time updates and continuous improvements, minimizing the risk of software failure and improving patient safety.
Treating SaMD as a software project enables developers to create software items that integrate seamlessly with medical systems, offering the flexibility to build custom medical device software tailored to specific healthcare needs.

Evgeniy Altynpara is a CTO and member of the Forbes Councils’ community of tech professionals. He is an expert in software development and technological entrepreneurship and has 10+years of experience in digital transformation consulting in Healthcare, FinTech, Supply Chain and Logistics
Give us your impressions about this article
Give us your impressions about this article